Management system guidance
Quality manual
Benefits of keeping your quality manual
The quality manual is often described as a top-level document. When deciding to keep your manual, it essential to take guidance from your accreditation body, suppliers, employees, customers, and other external stakeholders.
In business we are often distracted and can lose focus on what is important, the quality manual and the management system help to keep us focused.
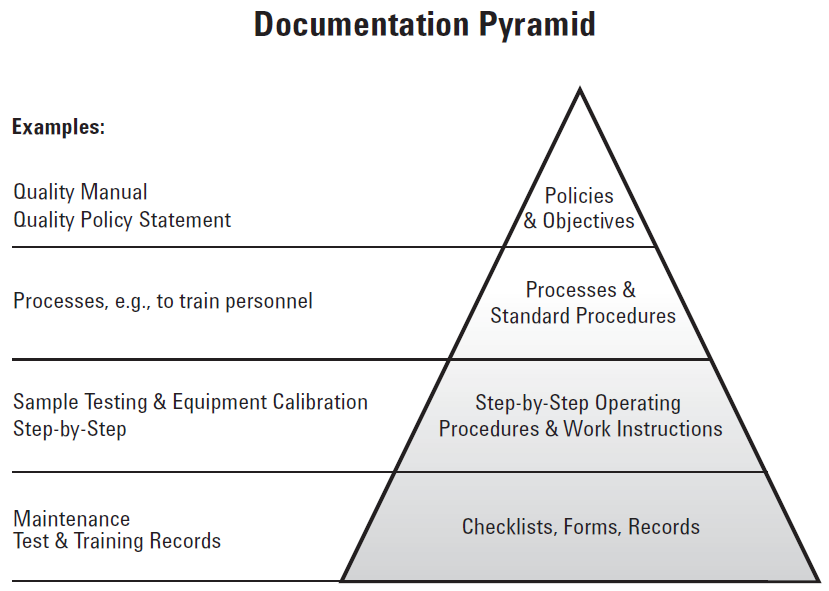
The quality manual describes the quality management system in accordance with the stated quality policy and objectives, while the procedures describe the processes and activities required to implement the quality management system.
Although ISO 9001 no longer requires the quality manual as a mandatory document, it is perhaps still the most important part of any modern quality management system.
Organizations often address the requirements of the standards by preparing a management system manual and by implementing procedures to control processes. In reality, it is still important still to document the important parts of the business, especially areas with the highest risk
Many businesses and operators of mature quality systems recognize the added value these elements bring to their operations and their quality management system. The quality manual, the polices, processes and procedures are all about what your company has decided is important to ensure they can provide the services and products that continually meet customer requirements, deliver satisfaction and for the business to meet its own targets and objectives.
The quality manual provides the scope of the management system (e.g. what the business does, wants, expects, etc.). Also, the manual contains an overview of management's and employee responsibilities as well as conformity statements applicable to the ISO 9001:2015 causes that are contained and supported by your management system.
The management system processes and procedures provide detailed requirements for each of your key processes with the intent to specify who does what, when, where, how the process, action, or task is performed, and what documentation is used to verify that all required the quality related activities have been executed as required.
Quality manual basics
Ensure the quality manual addresses all relevant requirements of ISO 9001, and that any non-applicable requirements from Section 8, Operation; are documented in the scope of the quality manual as appropriate. Operational procedures should be included or referenced in the quality manual, and the interaction between the processes of the quality system should be documented (e.g. process map, flowcharts, etc.).
The quality manual is often used as the normative basis of reference to international standards, and when used internally provides an overview of the ISO 9001:2015 requirements and processes. All management system documents should be properly controlled. For example, each change should be authorized and logged, and the updated document should get a new revision number or code.
Why have a manual if none is required for accreditation? You can document the procedures, scope, policy and anything else your business and the standard requires. You could create a booklet-sized resource in which the QMS documents are referenced, or even as a series tabs in a spreadsheet document, stored on a shared drive.
If you use a process map, the procedure references could be listed there. The procedures could be referenced as part of the actions to mitigate risks with in your risk-register. There are many practical methods to replace your quality manual!
Communicating the quality manual
Communicating your quality manual via the intranet or internet actively encourages engagement with stakeholders, employees, customers, suppliers and other interested parties. As a top-level, controlled document, the quality manual is often used as a business and marketing tool with the following indispensable benefits:
- Communicates business strategy;
- Defines how customer needs are identified, met and monitored;
- Marketing tool for promoting the organization and communicating its quality management system externally;
- A tool to establish and imbed organizational credibility and accountability;
- Documents management system scope;
- Documents management system processes;
- Communicates sequence and interaction;
- Communicates the quality policy;
- Communicates the quality objectives;
- Relevance to products and services, and sector;
- References documented information;
- Outlines core expectations;
- Attracts pre-qualification offers;
- Documenting and explaining exclusions;
- Alignment and compliance to standards and regulations;
- Certification/registration and Pre-audit reviews;
- Well-defined procedures and processes
- Supplier audit reference;
- Useful induction tool for new staff;
- Greater visibility of quality assurance for products and services.
Quality manuals allow you to demonstrate the quality of what you do for your customers, employees and stakeholders and will help embed best practice into your organization.
If you're looking to purchase a quality manual, our quality manuals, procedures and audit checklists are trusted by thousands of small businesses and globally recognized brands to reduce the risk of non-conformances and corrective action during the implementation, operation and certification of an ISO 9001 quality management system.
Quality manual scope
The quality manual provides a general description of your organization's QMS and the various processes employed within its scope. It adds value by helping the process owners to understand their roles within the management system. So, create a manual that is more user-friendly than auditor-friendly since a well designed quality manual would address the needs of employees and also the expectations of customers.
The scope of your quality manual should capture the structure and interactions of your business and its quality management system, delineate authorities, inter-relationships and responsibilities of personnel, provide reference to the procedures, forms, reports, process and activities that it describes.
The quality manual demonstrates implementation of the ISO 9000:2015 quality management principles and how they are adopted into daily operations as a way to provide a foundation for detecting and preventing non-conformities and continually improving.
Need a quality manual template?
Our ISO 9001 quality manual template, procedures, forms, and audit checklists provide the robust framework required for implementing a compliant, process-based quality management system. It is very comprehensive and goes into depth on every section of the ISO 9001:2015 standard.
Optional guidance documents and additional procedure templates are available to help you meet the quality management requirements of ISO 9001:2015, whatever the size and scope of your operations!
By developing the quality manual and procedures to align with the concepts, guidelines and terminology given in ISO 9000:2015, ISO 9004:2018 and ISO 19011:2018, the quality manual templates and procedures deliver clear, concise and well-documented content that provides long-term value for money!
Quality manual template formatting and compatibility
The editable quality manual templates are supplied in .docx and .xlsx formats, while the guidance documents are supplied .pdf format. They are compatible with Microsoft Office and Adobe Acrobat for Windows or MAC and are compatible with SharePoint, Microsoft Office 365, and all proprietary digital storage solutions. The quality manual templates are supplied as .zip files for fast download (3.9 to 9.2Mb) | extracted .docx and .pdf file sizes are between 1.5 and 8.5Mb.
Quality manual template example downloads
Download examples of our quality management system templates in .pdf format and see how they could help you save time and money!
Individual QMS templates
Also available are individual templates from our ISO 9001:2015 quality management system, including the quality manual template, internal audit checklists, supplier audit checklists, comprehensive guidance, procedures and forms, and gap analysis checklists. Perfect for updating your existing quality management system manual! Field audited and certified by Certification Bodies such as BSI, TUV and UKAS, our quality manual templates deliver clear, concise and well-documented content that provides long-term relevance and value for money.
Internal Auditing
~ €57.00 EUR, $67.14 USD, $97.09 CAD, $100.34 AUD
Internal audit checklists with audit result charts to help assess, monitor, and maintain compliance
- Internal audit checklist
- Internal audit programme
- Process audit checklist
- Email support
Supplier Auditing
~ €57.00 EUR, $67.14 USD, $97.09 CAD, $100.34 AUD
Supplier audit checklist and evaluation questionnaire with automatic results charts
- Supplier Audit Checklist
- Supplier Self-Evaluation
- Supplier Corrective Action Procedure
- Email support
Gap Analysis
~ €57.00 EUR, $67.14 USD, $97.09 CAD, $100.34 AUD
Gap analysis checklist, gap analysis action plan, with presentations and guidance
- Gap Analysis Action Plan
- Gap Analysis Checklist
- Gap Analysis Guidance
- Email support
Quality Manual
~ €57.00 EUR, $67.14 USD, $97.09 CAD, $100.34 AUD
46-page, professionally formatted quality manual template, with an editable process interaction map
- 46-page, quality manual template
- Follows the headings of ISO 9001
- Fully editable text and diagrams
- Auditing, and QMS presentations
- Email support
QMS Guidance
~ €170.99 EUR, $201.44 USD, $97.09 CAD, $301.05 AUD
With plenty of practical examples, the guidance documents describe the essential QMS elements
- 68-page Clause-by-clause guide
- 16-page Auditing guide
- 12-page Gap analysis guide
- 18-page Corrective action guide
- Auditing, and QMS presentations
Procedures & Forms
~ €170.99 EUR, $201.44 USD, $97.09 CAD, $301.05 AUD
10 quality management procedures with 10 turtle diagrams and 7 process maps, and 28 forms
- 10 Quality procedures
- 10 Turtle diagrams
- 28 Reports and forms
- 7 Process maps
- Email support
When you're ready to order, click the quality manual template's 'Get' button to open the order page. The template's download link will become available automatically after completing payment
Payment methods
International customers are welcome to pay using your PayPal account, or with your credit or debit card, in your currency. PayPal will automatically convert your payment, we can accept payments from buyers from 190 different countries!
Trusted by businesses worldwide
To experience real, long-term benefits, our ISO management system templates have continually evolved and supported our client's transition from different revisions of ISO 9001, including the 2000 and 2008 revisions, and presently; the 2015 revision. Updated to address the 2024 climate change amendments.
Since then, we have helped over 50,000 small businesses and globally recognized brands show strong governance by implementing their own ISO management system, we can help you too.
Certification Bodies such as BSI and UKAS, as well as independent Consultants and External Auditors, have commented upon the high-level of detail and excellent presentation standard. 'A practical guide to producing your own quality management system'. Cranfield University, UK.
Join thousands of small businesses and globally recognized brands who trust our management system resources, solutions, and tools to help them navigate complex compliance challenges, and achieve their certification goals in diverse business sectors ranging from:
✓ |
Consultancies | ✓ |
Aviation Services |
✓ |
Engineering |
✓ |
County, City & State Councils |
✓ |
Manufacturing |
✓ |
Printing & Publishing |
✓ |
Colleges, Universities & Students |
✓ |
Oil & Gas |
✓ |
Retail & Distribution |
✓ |
Digital Media & IT |
✓ |
Marine & Shipping |
✓ |
Construction & Subcontractors |
✓ |
Pharmaceuticals |
✓ |
Biotechnology |
✓ |
Private & State Healthcare |
✓ |
Government Agencies |
✓ |
Finance & Insurance |
✓ |
Renewable Energy |
Want to know more?
- Read our customer's feedback
- Client list - who's using our templates?
- How the templates are formatted and download examples
- Why we use turtle diagrams and process maps
- What's the difference between a process and a procedure?
- About documented information
Free management system interpretation guidance
Planning - Context, leadership and management system planning
This is the 'Plan' part of the PDCA process. Establish the objectives and processes necessary to deliver results in accordance with customer requirements and the organizational policies. This is often implemented using stated objectives, work instructions or procedures as required for consistent process output.
Doing - Support and operations
This is the 'Do' part of the PDCA process. Ensure the availability of resources and information necessary to support the operation and monitoring of your processes. This may be through management review or other methods that define resource requirements.
Checking - monitoring, measuring, analysing and evaluating
This is the 'Check' part of the PDCA process. Monitor, measure and analyse process performance. Monitor and measure processes and products against policies, objectives and requirements, and report the results. The methods employed and the timing of such analysis should be based upon priorities established by the organization.
Acting - implementing improvement actions
This is the 'Act' part of the PDCA process. Implement the actions necessary to achieve the planned results, and for the continual improvement of those processes. Auditors will expect to see evidence that corrective action is taken when measurable objectives and performance indicators fall below target or a pre-defined action level.
SSL certification
A certificate guarantees the information your internet browser is receiving now originates from the expected domain - https://www.iso9001help.co.uk. It guarantees that when you make a purchase, sensitive data is encrypted and sent to the right place, and not to a malicious third-party.
Free PDCA guidance
ISO Navigator™ is our FREE online training tool that shows you how to apply the principles of PDCA to your operations. We also offer many helpful templates that get you on the road to documenting your management system, please visit the download page.